Traditionally, multi-focal breast cancer results in mastectomy. The Alliance Trial offers a paradigm shift in surgical options available for multi-focal breast cancer patients in the context of adjuvant chemotherapy. In the trial, patients with multi-focal disease (2 or 3 tumors) who underwent breast conservation surgery (BCS) were found to have similar outcomes to patients undergoing mastectomy. BCS for large volume tumors (>30%) has been cited as having a high potential for cosmetic defect, and hence represents a typical upper limit for potential tissue removal in BCS.
Here, we evaluated a patient cohort to better understand the economic impact of the Alliance trial and further categorize patients that would most benefit without suffering cosmetic impact. We then extrapolated these findings using national breast cancer statistics to help establish broad cost estimates. We employed a novel computational technology to quantify the ratio of tumor size to breast tissue volume.
Alliance Trial Criteria
Inclusion Criteria
• Mastectomy
• Women age ≥ 40
• 2 or 3 foci of breast cancer
• At least one foci of invasive disease
• ≥ 2 cm normal tissue between lesions
• No more than 2 quadrants with disease
• cN0 or cN1 disease
Exclusion Criteria
• Focus of disease > 5 cm on imaging
• Bilateral breast cancer
• Prior ipsilateral breast cancer
• Known BRCA 1/2 mutations
• Neoadjuvant therapy
• Men
Methods
Using a publicly available, single site cohort (n=243, DUMC) of breast cancer patients that underwent mastectomy, we segmented the tumors using our TumorSight Viz software platform. This platform uses artificial intelligence to segment the tumor and surrounding tissues and allows for a volumetric and morphologic assessment in 3D space. We then applied relevant inclusion/exclusion criteria from the Alliance Trial to the cohort (Saha et al, 2018). In trial eligible patients, we used TumorSight Viz to create a convex hull (CH) around the multi-focal disease using dilations of 1 cm and 2 cm.
The volume of the CH, corresponding to proposed surgical extirpation, and the overall breast volume (BV) were then computationally assessed in 3D. The ratio of CH to BV (CH:BV) was calculated and a cutoff of 30% (high potential for cosmetic deformity) was applied. A cost analysis was then carried out. We determined the aggregate per annum savings that could potentially be realized by transforming a subset of mastectomies to BCS by tabulating total costs of mastectomy+reconstruction vs. BCS+WBI (whole-breast irradiation), as well as adjusted for relative rates of adjuvant therapy across the nationwide patient population.
Results
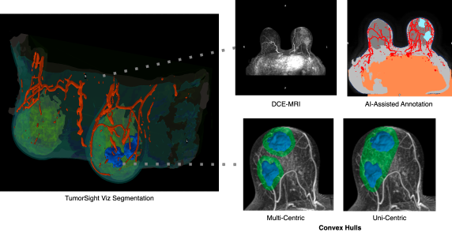
Figure 1. Multifocal breast cancer visualization in TumorSight Viz.
Adjuvant mastectomy patients eligible for BCS:
- Percent of eligible patients estimated using Duke cohort, based on Alliance Trial criteria: 47 of 243 (19.3%)
Tumor characteristics with CH:BV <30%
- 1 cm margin: 68%
- 2 cm margin: 56%
Overall eligibility for BCS in adjuvant mastectomy patients
- 19.3% of 68% = 13.1% with a 1 cm margin
- 19.3% of 56% = 10.8% with a 2 cm margin
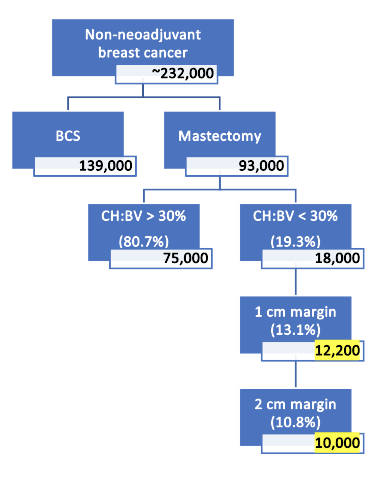
There will be an estimated ~280,000 newly-diagnosed cases of breast cancer in 2023. From this, we calculated estimated number of mastectomy and BCS in the absence of neoadjuvant therapy assuming an approximate rate of 20% for neoadjuvant therapy.
From this resulting ~232,000 breast cancer diagnoses, we applied Alliance Trial inclusion / exclusion criteria, along with volumetric statistics based on our findings from the DUMC cohort.
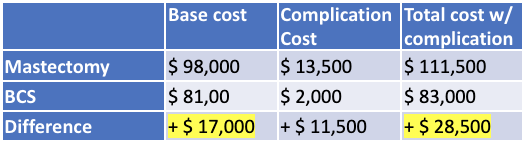
2023 costs were estimated by adjusting for inflation against 2014 costs (27% increase).

To determine the potential range of cost savings enabled by enhanced usage of BCS over mastectomy, we multiplied the number of patients that could be shifted from mastectomy to BCS by the relative excess cost incurred by mastectomy. Both the estimated number of mastectomies as well as the cost of surgery (with or without complication) have upper and lower bounds, which are factored into the overall cost range.
Conclusions
Our economic analysis of BCS vs. mastectomy revealed an estimated $17,000 – $28,500 cost savings for patients with private insurance, suggesting that both decreased costs and improved quality-of-life can be mutually aligned. By assessing the nationwide number of patients receiving adjuvant therapy for breast cancer, alongside the percentage potentially eligible for BCS using Alliance Trial Guidelines and a minimal potential for cosmetic defect (CH:BV < 30%), we estimate that BCS conversion from mastectomy offers to provide a net savings of $170-350 million annually.
References
- Cost and Complications of Local Therapies for Early-Stage Breast Cancer. J Natl Cancer Inst. 2017.
- CA A Cancer Journal for Clinicians. ACS Journals. Breast Cancer Statistics, 2022.
- Analysis of a Trend Reversal in US Lumpectomy Rates from 2005 Through 2017 Using 3 Nationwide Data Sets. JAMA Surgery. 2022.
About Us
SimBioSys is a software company deploying a combination of biophysical modeling and artificial intelligence to revolutionize precision cancer care. Our portfolio of software applications enables individualized treatment planning, accelerated drug development, clinical trial optimization and comprehensive biomarker development.

